
Chemistry, 17.03.2020 20:00, davfar334p47luq
Calcium carbonate is an "insoluble salt". But the solubility of CaCO3 can be increased substantially by acidifying the solution. Write all the relevant equilibrium equations and explain why adding acid will increase the solubility of calcium carbonate.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:10, dontcareanyonemo
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
Do you know the correct answer?
Calcium carbonate is an "insoluble salt". But the solubility of CaCO3 can be increased substantially...
Questions in other subjects:

Mathematics, 06.01.2020 21:31

History, 06.01.2020 21:31







Biology, 06.01.2020 21:31


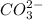 is converted to
is converted to 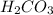 in acidic solution.
in acidic solution.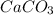 dissociates in solution to produce
dissociates in solution to produce 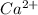 and
and 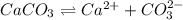
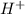 and gets converted to
and gets converted to 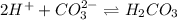 So,
So, 




