
Chemistry, 17.03.2020 19:24, bobbyhill24
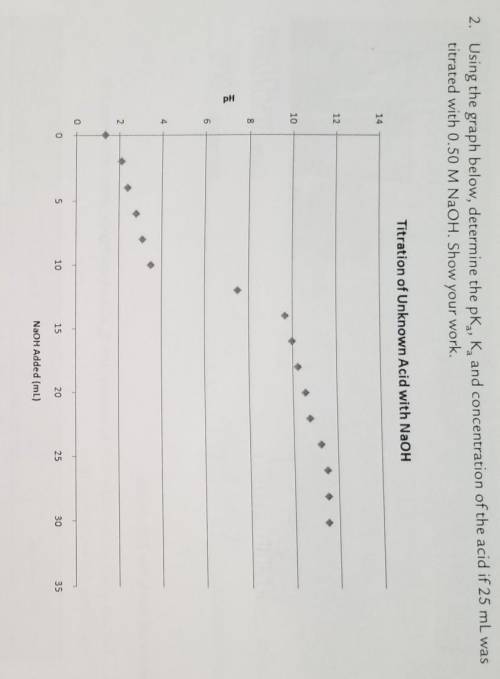
Using the graph below, determine the pKa, Ka, and concentration of the acid if 25 mL was titrated with 0.50 M NaOH. Show your work.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1


Chemistry, 23.06.2019 08:00, kathrynpuppies201716
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
Do you know the correct answer?
Using the graph below, determine the pKa, Ka, and concentration of the acid if 25 mL was titrated wi...
Questions in other subjects:

Mathematics, 27.03.2021 23:20

History, 27.03.2021 23:20

Business, 27.03.2021 23:20



Physics, 27.03.2021 23:20

Computers and Technology, 27.03.2021 23:20

Mathematics, 27.03.2021 23:20

Business, 27.03.2021 23:20

Mathematics, 27.03.2021 23:20






