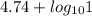Acetic acid has a Ka of 1.8*10^-5. Three acetic acid/ acetate buffer solutions, A, B, and C, wer made using varying concentrations: 1. [acetic acid] ten times greater than [acetate] 2. [acetate] ten times greater than [acetic acid] 3. [acetate] = [acetic acid] Match each buffer to the expected pH pH = 3.74 pH= 4.74 pH = 5.74

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
Do you know the correct answer?
Acetic acid has a Ka of 1.8*10^-5. Three acetic acid/ acetate buffer solutions, A, B, and C, wer mad...
Questions in other subjects:

Mathematics, 12.04.2021 23:40





Social Studies, 12.04.2021 23:40





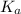 for acetic acid is
for acetic acid is 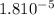 . And, its
. And, its  value will be calculated as follows.
value will be calculated as follows.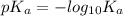
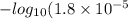
![pK_{a} + log \frac{[Salt]}{[Acid]}](/tpl/images/0550/7017/81f72.png)
![\frac{[\text{Acetate}]}{[\text{Acetic acid}]} = \frac{1}{10}](/tpl/images/0550/7017/c529e.png)
![pK_{a} + log \frac{[Acetate]}{[\text{Acetic Acid}]}](/tpl/images/0550/7017/a17de.png)
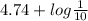
![\frac{[Acetate]}{\text{Acetic acid}}](/tpl/images/0550/7017/7d291.png) =
= 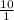
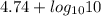
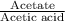 = 1
= 1