
Chemistry, 17.03.2020 04:31, lobatospitones
Determine the rate constant for each of the following fi rst-order reactions, in each case expressed for the rate of loss of A: (a) A S B, given that the concentration of A decreases to one-half its initial value in 1000. s; (b) A S B, given that the concentration of A decreases from 0.67 molL1 to 0.53 molL1 in 25 s; (c) 2 A S B C, given that [A]0 0.153 molL1 and that after 115 s the concentration of B rises to 0.034 molL1

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
Do you know the correct answer?
Determine the rate constant for each of the following fi rst-order reactions, in each case expressed...
Questions in other subjects:

Mathematics, 12.12.2019 23:31

Mathematics, 12.12.2019 23:31

English, 12.12.2019 23:31


Mathematics, 12.12.2019 23:31


Mathematics, 12.12.2019 23:31



English, 12.12.2019 23:31


![[A]=[A]_o\times e^{-k\times t}](/tpl/images/0550/2081/d7033.png)
![[A]_o](/tpl/images/0550/2081/9caf5.png) = initial concentration of reactant
= initial concentration of reactant ![[A_o]=x](/tpl/images/0550/2081/aecde.png)
![[A]=\frac{x}{2}](/tpl/images/0550/2081/f7c20.png)
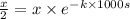
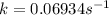
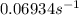 .
.![[A_o]=0.67 mol/L](/tpl/images/0550/2081/74ad9.png)
![[A]=0.53 mol/L](/tpl/images/0550/2081/d3d0a.png)
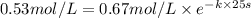
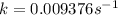
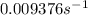 .
.![[A_o]=0.153 mol/L](/tpl/images/0550/2081/3406c.png)
![[A]=?](/tpl/images/0550/2081/8be21.png)
![[B]=0.034 mol/L](/tpl/images/0550/2081/2fbaf.png)
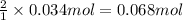 of A
of A![[A]=0.153 mol/L-0.068 mol/L=0.085 mol/L](/tpl/images/0550/2081/97bbf.png)
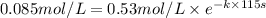
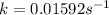
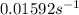 .
.



