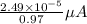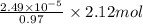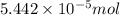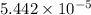Chemistry, 17.03.2020 04:40, hannahking1869
(a) [10 pts] Using an electrochemical method, you measured the ascorbic acid of a 30.0 mL watermelon juice sample. A detector signal of 2.12 uA was observed. A standard addition of 1.00 mL of 24.9 mM ascorbic acid standard increased the current to 3.09 uA. Find the concentration of vitamin C in the watermelon juice.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
Do you know the correct answer?
(a) [10 pts] Using an electrochemical method, you measured the ascorbic acid of a 30.0 mL watermelon...
Questions in other subjects:




Business, 29.09.2019 05:20





Geography, 29.09.2019 05:20

Social Studies, 29.09.2019 05:20


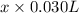
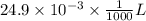
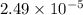 mole
mole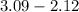

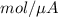 will be equal to as follows.
will be equal to as follows.