
Chemistry, 17.03.2020 03:18, violetagamez2
Sodium sulfate is slowly added to a solution containing 0.0500 M Ca 2 + ( aq ) and 0.0300 M Ag + ( aq ) . What will be the concentration of Ca 2 + ( aq ) when Ag 2 SO 4 ( s ) begins to precipitate? Solubility-product constants, K sp , can be found in the chempendix.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Do you know the correct answer?
Sodium sulfate is slowly added to a solution containing 0.0500 M Ca 2 + ( aq ) and 0.0300 M Ag + ( a...
Questions in other subjects:





Business, 23.10.2019 22:00






![[Ca^{2+}]](/tpl/images/0550/0535/17576.png) ion is, 0.00371 M
ion is, 0.00371 M![[SO_4^{2-}]](/tpl/images/0550/0535/43b69.png) ion.
ion.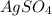 will be:
will be: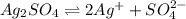
![K_{sp}=[Ag^{+}]^2[SO_4^{2-}]](/tpl/images/0550/0535/80963.png)
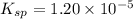
![1.20\times 10^{-5}=(0.0300)^2\times [SO_4^{2-}]](/tpl/images/0550/0535/d8931.png)
![[SO_4^{2-}]=0.0133M](/tpl/images/0550/0535/cd404.png)
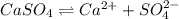
![K_{sp}=[Ca^{2+}][SO_4^{2-}]](/tpl/images/0550/0535/958f2.png)
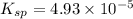
![4.93\times 10^{-5}=[Ca^{2+}]\times (0.0133)](/tpl/images/0550/0535/7fa1a.png)
![[Ca^{2+}]=0.00371M](/tpl/images/0550/0535/7d329.png)




