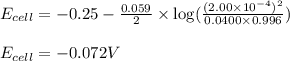Calculate the theoretical potential of the following cell. Indicate whether the reaction will proceed spontaneously in the direction considered (oxidation on the left, reduction on the right) or whether an external voltage source is needed to force this reaction to occur.
Pt, H2(757 torr)|HCl(2.00×10-4 M) parallel to Ni2+(0.0400 M)|Ni
the answer is
-0.072 V; External voltage needed
can anyone explain to me how i get this answer ?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 03:30, vaehcollier
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 03:50, timothymoles
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
Do you know the correct answer?
Calculate the theoretical potential of the following cell. Indicate whether the reaction will procee...
Questions in other subjects:

Chemistry, 03.04.2020 09:26



History, 03.04.2020 09:26


Biology, 03.04.2020 09:27



History, 03.04.2020 09:28

English, 03.04.2020 09:28


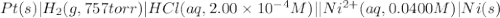
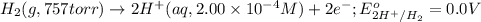
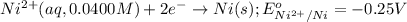
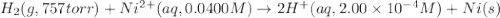
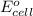 of the reaction, we use the equation:
of the reaction, we use the equation: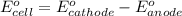
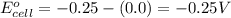
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[H^{+}]^2}{[Ni^{2+}]\times p_{H_2}}](/tpl/images/0549/9866/bc143.png)
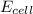 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[H^{+}]=2.00\times 10^{-4}M](/tpl/images/0549/9866/69105.png)
![[Ni^{2+}]=0.0400M](/tpl/images/0549/9866/57673.png)
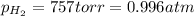 (Conversion factor: 1 atm = 760 torr)
(Conversion factor: 1 atm = 760 torr)