
Chemistry, 17.03.2020 01:09, haileeattaway
The reaction between NO2 and CO to produce NO and CO2 is believed to occur via two steps: step 1: NO2 + NO2 → NO + NO3 step 2: NO3 + CO → NO2 + CO2 The experimental rate law is rate = k[NO2]2 (a) Write the equation for the overall reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
Do you know the correct answer?
The reaction between NO2 and CO to produce NO and CO2 is believed to occur via two steps: step 1: NO...
Questions in other subjects:

Health, 11.09.2021 14:00

Mathematics, 11.09.2021 14:00

Mathematics, 11.09.2021 14:00

Chemistry, 11.09.2021 14:00




Arts, 11.09.2021 14:00



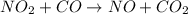
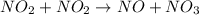
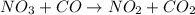
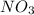 is produced in the first step and subsequently consumed in second step. Hence,
is produced in the first step and subsequently consumed in second step. Hence, 




