
Chemistry, 17.03.2020 00:54, kprincess16r
A certain metal M crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The lattice constant (a, the edge of the unite cell) has been measured by X-ray crystallography to be 360. pm. Calculate the radius of an atom of M. Be sure your answer has 3 significant digits, and be sure it has the correct unit symbol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2


Do you know the correct answer?
A certain metal M crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The...
Questions in other subjects:

History, 22.06.2019 17:30










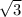 /4
/4



