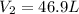A weather balloon is inflated to a volume of 28.6 L at a pressure of 737 mmHg and a temperature of 26.8 ∘C. The balloon rises in the atmosphere to an altitude where the pressure is 385 mmHg and the temperature is -16.3 ∘C. Assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
Do you know the correct answer?
A weather balloon is inflated to a volume of 28.6 L at a pressure of 737 mmHg and a temperature of 2...
Questions in other subjects:




Mathematics, 06.01.2021 23:20

History, 06.01.2021 23:20

Mathematics, 06.01.2021 23:20

Mathematics, 06.01.2021 23:20

Mathematics, 06.01.2021 23:20

Mathematics, 06.01.2021 23:20

English, 06.01.2021 23:20



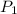 = initial pressure of gas = 737 mm Hg
= initial pressure of gas = 737 mm Hg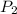 = final pressure of gas = 385 mm Hg
= final pressure of gas = 385 mm Hg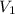 = initial volume of gas = 28.6 L
= initial volume of gas = 28.6 L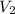 = final volume of gas = ?
= final volume of gas = ?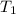 = initial temperature of gas =
= initial temperature of gas = 
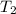 = final temperature of gas =
= final temperature of gas =