

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
Do you know the correct answer?
Small quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KClO 3 (...
Questions in other subjects:


Mathematics, 18.11.2020 21:00


Spanish, 18.11.2020 21:00

History, 18.11.2020 21:00

Mathematics, 18.11.2020 21:00

Advanced Placement (AP), 18.11.2020 21:00

Health, 18.11.2020 21:00



 produced = 32 g
produced = 32 g as:-
as:-

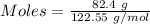


 moles of oxygen gas
moles of oxygen gas



