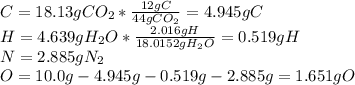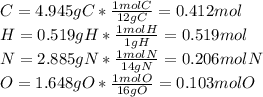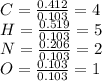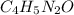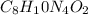Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, ashgold324
If this equation was completed which statement would it best support
Answers: 2


Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2
Do you know the correct answer?
Caffeine, a molecule found in coffee, tea, and certain soft drinks, contains C, H, O, and N. Combust...
Questions in other subjects:



Physics, 22.09.2019 02:50

English, 22.09.2019 02:50


Mathematics, 22.09.2019 02:50