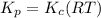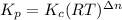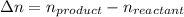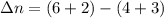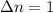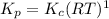Consider the following chemical equilibrium: 4NH3+3O2=2N2+6H2O Now write an equation below that shows how to calculate from for this reaction at an absolute temperature . You can assume is comfortably above room temperature. If you include any common physical constants in your equation be sure you use their standard symbols, found in the ALEKS Calculator.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1



Chemistry, 23.06.2019 14:30, ajahbraun
Recognizing the properties of water water has a "bent" geometry. which explanation does not explain why? o water's oxygen has unbonded electron pairs that repel each other. water can form hydrogen bonds. electrons are evenly distributed in the water molecule. do ne
Answers: 3
Do you know the correct answer?
Consider the following chemical equilibrium: 4NH3+3O2=2N2+6H2O Now write an equation below that show...
Questions in other subjects:

Chemistry, 22.01.2021 20:10



English, 22.01.2021 20:10

Mathematics, 22.01.2021 20:10


Mathematics, 22.01.2021 20:10


Mathematics, 22.01.2021 20:10

History, 22.01.2021 20:10


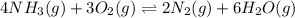
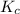 will be,
will be,![K_c=\frac{[N_2]^2[H_2O]^6}{[NH_3]^4[O_2]^3}](/tpl/images/0549/5664/b62d0.png)
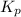 will be,
will be,