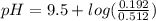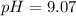Chemistry, 16.03.2020 23:01, thanks5640
A buffer solution contains 0.353 M ammonium bromide and 0.352 M ammonia. If 0.0200 moles of hydrochloric acid are added to 125 mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume change does not change upon adding hydrochloric acid)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 19:00, lizbeth232001
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
Do you know the correct answer?
A buffer solution contains 0.353 M ammonium bromide and 0.352 M ammonia. If 0.0200 moles of hydrochl...
Questions in other subjects:


Mathematics, 08.01.2021 23:20


English, 08.01.2021 23:20

Mathematics, 08.01.2021 23:20

Mathematics, 08.01.2021 23:20

Mathematics, 08.01.2021 23:20




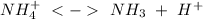
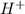 of the hydrochloric acid (
of the hydrochloric acid (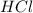 ) will interact with the base of the buffer system (
) will interact with the base of the buffer system (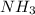 ) to produce more acid (
) to produce more acid (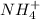 ), so:
), so: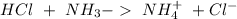
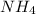 will increase. The next step then would be the calculation of the moles of the acid and base in the buffer system. So:
will increase. The next step then would be the calculation of the moles of the acid and base in the buffer system. So: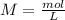
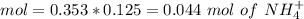
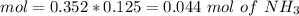
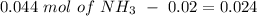
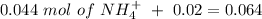
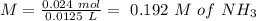
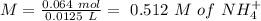
![pH=p{ K }_{ a }+log(\frac { { [A }^{ - }] }{ [HA] } )](/tpl/images/0549/5131/c1f49.png)