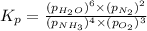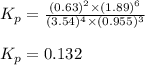Chemistry, 16.03.2020 22:22, fordalolz1553
Problem Page Question Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a flask with of ammonia gas and of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of water vapor to be . Calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to significant digits. Clears your work. Undoes your last action. Provides information about entering answers.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
Do you know the correct answer?
Problem Page Question Ammonia has been studied as an alternative "clean" fuel for internal combustio...
Questions in other subjects:


Mathematics, 15.07.2019 07:20

Mathematics, 15.07.2019 07:20

Mathematics, 15.07.2019 07:20


Mathematics, 15.07.2019 07:20

Mathematics, 15.07.2019 07:20

Mathematics, 15.07.2019 07:20

History, 15.07.2019 07:20


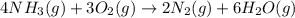
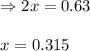
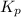 for above equation follows:
for above equation follows: