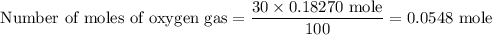Chemistry, 16.03.2020 21:24, nekobaby75
A 3.0-L gas mixture contains 30.% oxygen and 70.% nitrogen. Use the ideal gas law to determine the number of moles of oxygen at 2.0 atm and 400-K.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, antoninapride
Which statement justifies that phosphine (ph3) is a polar molecule?
Answers: 1


Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
Do you know the correct answer?
A 3.0-L gas mixture contains 30.% oxygen and 70.% nitrogen. Use the ideal gas law to determine the n...
Questions in other subjects:

History, 30.05.2020 09:58

Mathematics, 30.05.2020 09:58

Mathematics, 30.05.2020 09:58

Biology, 30.05.2020 09:58

Mathematics, 30.05.2020 09:58




Mathematics, 30.05.2020 09:58

Social Studies, 30.05.2020 09:58