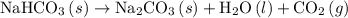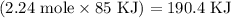Chemistry, 16.03.2020 21:15, msjsnell29
Given the following information: aHCO3(s)+85 kJ Na2CO3(s)+H2O(1) CO2(g) Calculate the amount of heat (ink) required to decompose 2.24 mol NaHCO3(s). You must show your work to receive credit .

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 23.06.2019 09:30, raymondmancilla123
The mass of a proton is approximately equal to the mass of
Answers: 1
Do you know the correct answer?
Given the following information: aHCO3(s)+85 kJ Na2CO3(s)+H2O(1) CO2(g) Calculate the amount of heat...
Questions in other subjects:




English, 26.09.2019 06:30

English, 26.09.2019 06:30

Mathematics, 26.09.2019 06:30



Mathematics, 26.09.2019 06:30

Biology, 26.09.2019 06:30


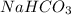 is shown below
is shown below