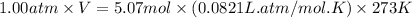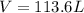Chemistry, 16.03.2020 21:05, chancecharles9oug353
Roasting galena [lead(II) sulfide] is an early step in the industrial isolation of lead. How many liters of sulfur dioxide, measured at STP, are produced by the reaction of 6.61 kg of galena with 154 L of oxygen gas at 220°C and 2.00 atm? Lead(II) oxide also forms.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Aidanjsauer
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3
Do you know the correct answer?
Roasting galena [lead(II) sulfide] is an early step in the industrial isolation of lead. How many li...
Questions in other subjects:


Mathematics, 30.03.2021 22:20

Geography, 30.03.2021 22:20


Mathematics, 30.03.2021 22:20

Mathematics, 30.03.2021 22:20



English, 30.03.2021 22:20

Mathematics, 30.03.2021 22:20


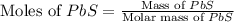
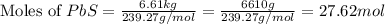

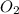 gas = 2.00 atm
gas = 2.00 atm

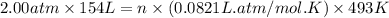


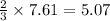 moles of PbS
moles of PbS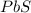 is an excess reagent because the given moles are greater than the required moles and
is an excess reagent because the given moles are greater than the required moles and