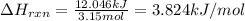3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution's temperature increases by 16.01°C. Calculate ∆H for the dissolution of the unknown solid. (The specific heat of the solution is 4.18 J/g・°C and the density of the solution is 1.20 g/mL).

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, KieraKimball
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 01:40, natannale
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
Do you know the correct answer?
3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution'...
Questions in other subjects:

Spanish, 13.05.2021 06:30

Health, 13.05.2021 06:30

Mathematics, 13.05.2021 06:30

Mathematics, 13.05.2021 06:30



Mathematics, 13.05.2021 06:30


Arts, 13.05.2021 06:30

Mathematics, 13.05.2021 06:30


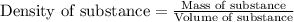
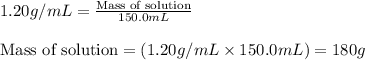
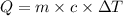
 = change in temperature = 16.01°C
= change in temperature = 16.01°C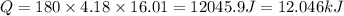
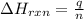
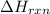 = enthalpy change of the reaction
= enthalpy change of the reaction