Chemistry, 16.03.2020 18:45, zeldawhite76
Morphine has the formula C17H19NO3. It is a base and accepts one proton per molecule. It is isolated from opium. A 0.682g sample of opium is found to require 8.92 mL of a 0.0116 M solution of sulfuric acid for neutralization. Assuming that morphine is the only base present in opium, calculate the mass (in grams) of morphine in the sample of opium.
Reaction equation: 2 C17H19NO3 + H2SO4 --> Product

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 05:30, brianrodriguez2005
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b. cns c. ans d. pns
Answers: 1
Do you know the correct answer?
Morphine has the formula C17H19NO3. It is a base and accepts one proton per molecule. It is isolated...
Questions in other subjects:



Mathematics, 03.09.2021 05:20

Biology, 03.09.2021 05:20


History, 03.09.2021 05:20

Advanced Placement (AP), 03.09.2021 05:20

Mathematics, 03.09.2021 05:20

Mathematics, 03.09.2021 05:20

Mathematics, 03.09.2021 05:20




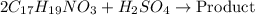
 of sulfuric acid will react with =
of sulfuric acid will react with =