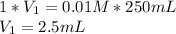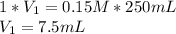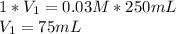The breaking buffer that we use this week contains 10mM Tris, pH 8.0, 150mM NaCl. The elution buffer is breaking buffer that also contains 300mM imidazole. Describe how the instructor made the 0.25L elution buffer for all the students this week given 500ml of 1M of Tris (121.1 g/mole) (pH8.0), 750ml of 5M NaCl (MW = 58.44 g/mole) and 350ml of 1M imidazole (68.08 g/mole). Show your calculations for credit.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
Do you know the correct answer?
The breaking buffer that we use this week contains 10mM Tris, pH 8.0, 150mM NaCl. The elution buffer...
Questions in other subjects:

Mathematics, 23.12.2020 07:00

Computers and Technology, 23.12.2020 07:00






Mathematics, 23.12.2020 07:00



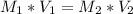 . It was the addition of these volumes altogether that make up the 0.25 L (i.e 250 mL) with water
. It was the addition of these volumes altogether that make up the 0.25 L (i.e 250 mL) with water