Chemistry, 16.03.2020 18:22, leannesmith90101
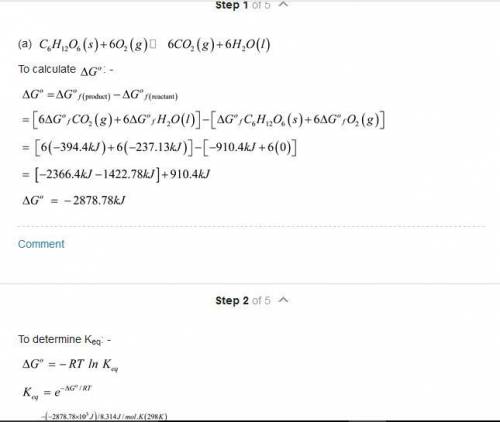
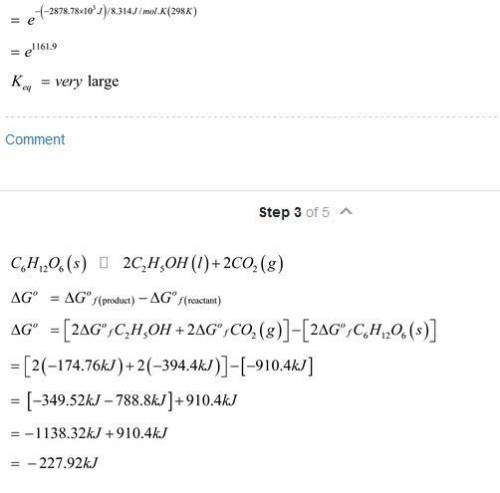
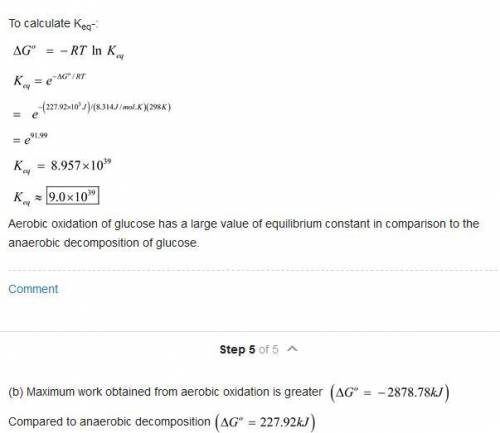
The oxidation of glucose (C6H12O6) in body tissue produces CO2 and H2O. In contrast, anaerobic decomposition, which occurs during fermentation, produces ethanol (C2H5OH) and CO2. Using data given in Appendix C, compare the equilibrium constants for the following reactions: C6H12O6(s)+6O2(g)⥫⥬6CO2(g)+6H2O(l)C 6H12O6(s)⥫⥬2C2H5OH(l)+2CO2(g) Compare the maximum work that can be obtained from these processes under standard conditions.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3



Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
Do you know the correct answer?
The oxidation of glucose (C6H12O6) in body tissue produces CO2 and H2O. In contrast, anaerobic decom...
Questions in other subjects:

Mathematics, 12.12.2020 16:50



Biology, 12.12.2020 16:50



History, 12.12.2020 16:50