

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1
Do you know the correct answer?
An unknown substance has a melting point of 1064°C, is insoluble in water, conducts electricity as a...
Questions in other subjects:

English, 03.06.2020 14:58

Advanced Placement (AP), 03.06.2020 14:58

Mathematics, 03.06.2020 14:58

Mathematics, 03.06.2020 14:58






Mathematics, 03.06.2020 14:58


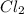 is a gas and it is made up of a non-metal. And, non-metals are poor conductors of heat and electricity therefore,
is a gas and it is made up of a non-metal. And, non-metals are poor conductors of heat and electricity therefore, 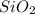 is a non-metallic compound therefore, it is unable to conduct electricity.
is a non-metallic compound therefore, it is unable to conduct electricity. 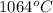 and it is insoluble in water and hard in nature. Also, it is able to conduct electricity in solid state.
and it is insoluble in water and hard in nature. Also, it is able to conduct electricity in solid state.



