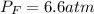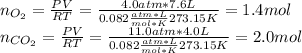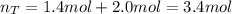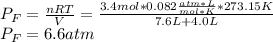Chemistry, 16.03.2020 18:05, eddsworldfrantic
A valve separates two tanks, one containing 7.6 liters of oxygen at 4.0 atmospheres and the other containing 4.0 liters of carbon dioxide at 11.0 atm. When the valve is opened and the two gases are allowed to come together, what is final pressure

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
Do you know the correct answer?
A valve separates two tanks, one containing 7.6 liters of oxygen at 4.0 atmospheres and the other co...
Questions in other subjects:





Mathematics, 08.10.2019 13:10


Mathematics, 08.10.2019 13:10

History, 08.10.2019 13:10