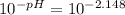Chemistry, 16.03.2020 18:08, jasminer257
N unknown weak acid, HA, it titrated with 0.6 M NaOH. The pH at the halfway point of this titration was found to be 4.215. If the initial pH of the weak acid solution (before titration) has a pH of 2.148, what was the concentration of the weak acid solution

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1


Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
Do you know the correct answer?
N unknown weak acid, HA, it titrated with 0.6 M NaOH. The pH at the halfway point of this titration...
Questions in other subjects:



Social Studies, 20.10.2021 14:10


Mathematics, 20.10.2021 14:10




Mathematics, 20.10.2021 14:10


 M
M