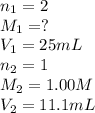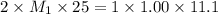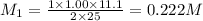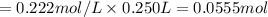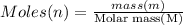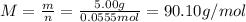Chemistry, 16.03.2020 17:02, staxeeyy767
A 5.00g quantity of a diprotic acid was dissolved in water and made up exactly 250 mL. Calculate the molar mass if the acid is 25.0 mL of this solution required 11.1 mL of 1.00 KOH for neutralization. Assume both protons of the acid were titrated.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 23.06.2019 01:00, breemills9552
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1

Chemistry, 23.06.2019 05:40, girlchamp654
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
Do you know the correct answer?
A 5.00g quantity of a diprotic acid was dissolved in water and made up exactly 250 mL. Calculate the...
Questions in other subjects:

Mathematics, 09.02.2021 21:00



SAT, 09.02.2021 21:00


English, 09.02.2021 21:00

Mathematics, 09.02.2021 21:00


Mathematics, 09.02.2021 21:00

English, 09.02.2021 21:00


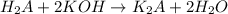
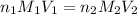 ( neutralization )
( neutralization )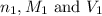 are the n-factor, molarity and volume of diprotic acid
are the n-factor, molarity and volume of diprotic acid 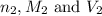 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.