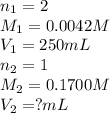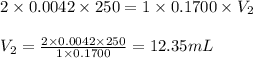Chemistry, 16.03.2020 16:37, DonovanBaily42
A chemist weighs out 0.0865 g of sulfurous acid, H2SO3, which is a diprotic acid into a 250. mL of volumetric flask and dilutes to the mark with distilled water. She plans to titrate the acid with 0.1700 M NaOH solution. Calculate the volume of NaOH solution in milliliters the student will need to add to reach the final equivalence point. (Molar mass of sulfurous acid = 82.079 g/moL)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2

Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 06:00, fjsdfj1284
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2

Chemistry, 23.06.2019 10:00, jdmXdude3140
How to draw a diagram to represent a calcium metal lattice?
Answers: 3
Do you know the correct answer?
A chemist weighs out 0.0865 g of sulfurous acid, H2SO3, which is a diprotic acid into a 250. mL of v...
Questions in other subjects:

Mathematics, 02.11.2020 21:40




Mathematics, 02.11.2020 21:40


Mathematics, 02.11.2020 21:40

English, 02.11.2020 21:40


Spanish, 02.11.2020 21:40


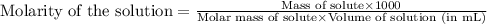

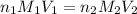
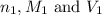 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 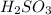
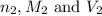 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.