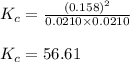Chemistry, 16.03.2020 16:29, jennemylesp19oy5
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture is heated to 425oC. At equilibrium, the concentration of I2is found to be 0.0210 M. Calculate Kcfor the following reaction at 425oC. H2(g) + I2(g) ⇄2 HI(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1


Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2
Do you know the correct answer?
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture i...
Questions in other subjects:



Biology, 31.01.2020 19:06

Mathematics, 31.01.2020 19:06

Mathematics, 31.01.2020 19:06


Mathematics, 31.01.2020 19:06


Physics, 31.01.2020 19:06


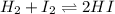
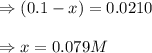
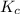 for above equation follows:
for above equation follows:![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0548/4996/62646.png)
![[HI]_{eq}=2x=(2\times 0.079)=0.158M](/tpl/images/0548/4996/cad3c.png)
![[H_2]_{eq}=(0.1-x)=(0.1-0.079)=0.0210M](/tpl/images/0548/4996/029c8.png)
![[I_2]_{eq}=0.0210M](/tpl/images/0548/4996/0be20.png)