
Balance the following chemical equation by the half-reaction method in acidic conditions. Show your work. P4 + NO3–→ H2PO4– + NO(A) What is the oxidized element? What is the reduced element?
(B) Write balanced half reactions for the oxidation reaction:
(C) Write balanced half reactions for the reduction reaction:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, yyyyyy290
Why are gamma rays not affected by a magnet as they pass over it? gamma rays are composed of only energy. gamma rays do not have enough mass to be affected. gamma rays do not have the right electrical charge to be affected. gamma rays move too fast for anything to affect their pathway.
Answers: 1


Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
Do you know the correct answer?
Balance the following chemical equation by the half-reaction method in acidic conditions. Show your...
Questions in other subjects:


Biology, 26.06.2019 18:30

History, 26.06.2019 18:30

Physics, 26.06.2019 18:30




Computers and Technology, 26.06.2019 18:30


English, 26.06.2019 18:30


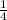 P4 + 4H2O ⇒ H2PO4- + 6H+ + 5e
P4 + 4H2O ⇒ H2PO4- + 6H+ + 5e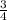 P4 + 5NO3- +2H+ + 2H2O⇒ 5NO+3H3PO4-
P4 + 5NO3- +2H+ + 2H2O⇒ 5NO+3H3PO4-



