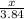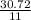Chemistry, 15.03.2020 02:28, poweradampower
The chemical reaction in this example is of environmental interest. Iron pyrite (Fesz) is often an impurity in
coal, and so burning this fuel in a power plant produces sulfur dioxide (SO), a major air pollutant.
Calculate the mass of sulfur dioxide (SO2) produced when 3.84 mol O2 is reacted with Fes, according to the
equation
4 FeSacs + 11 O.
- 2 Fe2O3(-) + S SO2(e)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
Do you know the correct answer?
The chemical reaction in this example is of environmental interest. Iron pyrite (Fesz) is often an i...
Questions in other subjects:

Mathematics, 01.12.2019 22:31

Mathematics, 01.12.2019 22:31

Mathematics, 01.12.2019 22:31

Mathematics, 01.12.2019 22:31


Geography, 01.12.2019 22:31



Mathematics, 01.12.2019 22:31

English, 01.12.2019 22:31


 =
=