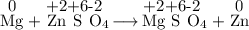Chemistry, 15.03.2020 02:21, jerseygirl6167
Consider the following reaction: Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s) Which of the following statements regarding this reaction is correct?
The sulfate ion is reduced.
Zinc is the reducing agent.
Magnesium is neither oxidized nor reduced.
Zinc gains two electrons.
Magnesium is the oxidizing agent.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, saleenhernandez83
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 00:50, alainacorkell6472
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
Do you know the correct answer?
Consider the following reaction: Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s) Which of the following statem...
Questions in other subjects:

History, 04.09.2020 19:01

Mathematics, 04.09.2020 19:01

History, 04.09.2020 19:01


Biology, 04.09.2020 19:01




Mathematics, 04.09.2020 19:01

Computers and Technology, 04.09.2020 19:01