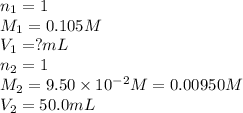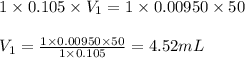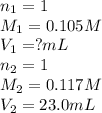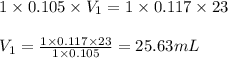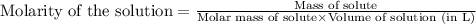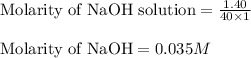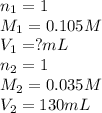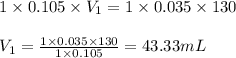Chemistry, 13.03.2020 22:21, WritingStar1313
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equivalence point?
a. 50.0 mL of 9.5010?2 M NaOH
b. 23.0 mL of 0.117 M NH3
c. 130 mL of a solution that contains 1.40 g of NaOH per liter

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
Do you know the correct answer?
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equ...
Questions in other subjects:


Mathematics, 08.01.2021 22:40

History, 08.01.2021 22:40

English, 08.01.2021 22:40



Mathematics, 08.01.2021 22:40


Mathematics, 08.01.2021 22:40

Mathematics, 08.01.2021 22:40


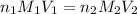
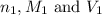 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid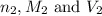 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base