
Chemistry, 13.03.2020 20:03, LilFreaky666
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (aq) + 3 H₂ (g); ΔH₁ = − 878.0 k J
HCl (g) ⟶ HCl (aq); ΔH₂ = − 74.8 k J
H₂ (g) + Cl₂ (g) ⟶ 2 HCl (g); ΔH₃ = − 1845.0 k J
MCl₃ (s) ⟶ MCl₃ ( aq ); ΔH₄ = − 497.0 k J
Use the given information to determine the enthalpy of the reaction
2 M (s) + 3 Cl₂ (g) ⟶ 2 MCl₃ (s).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1



Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
Do you know the correct answer?
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
Questions in other subjects:

Mathematics, 16.06.2021 14:00



Mathematics, 16.06.2021 14:00

Mathematics, 16.06.2021 14:00

Business, 16.06.2021 14:00


Physics, 16.06.2021 14:00



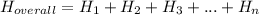 .
.




