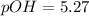Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, anarosa331hotmailcom
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1
Do you know the correct answer?
If a 95.27 mL sample of acetic acid (HC2H3O2) is titrated to the equivalence point with 79.06 mL of...
Questions in other subjects:

Chemistry, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01


Chemistry, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Biology, 16.10.2020 18:01


Chemistry, 16.10.2020 18:01


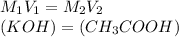
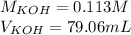
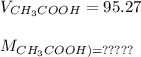
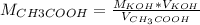
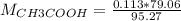
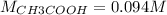
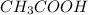 =
= 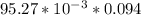
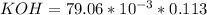

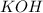 ----->
-----> 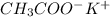
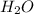
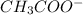 ion =
ion = 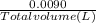
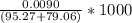
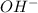
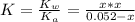
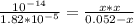
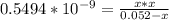
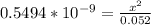

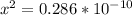
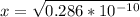
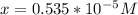
![[OH] = x =0.535*10^{-5}](/tpl/images/0545/6807/399d1.png)
![pOH = -log[OH^-]](/tpl/images/0545/6807/12649.png)
![pOH = -log[0.535*10^{-5}]](/tpl/images/0545/6807/26e4d.png)