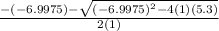Chemistry, 12.03.2020 21:40, daniellaZemira
Suppose a 250.0 mL flask is filled with 1.3 mol of I2 and 1.0 mol of HI. The following reaction becomes possible:
H2 (g) +I2 (g) ⇆ 2HI (g)
The equilibrium constant for this reaction is 0.983 at the temperature of the flask.
Calculate the equilibrium molarity of HI. Round your answer to two decimal places.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
Do you know the correct answer?
Suppose a 250.0 mL flask is filled with 1.3 mol of I2 and 1.0 mol of HI. The following reaction beco...
Questions in other subjects:

History, 14.07.2020 22:01

Mathematics, 14.07.2020 22:01

Mathematics, 14.07.2020 22:01

History, 14.07.2020 22:01

Mathematics, 14.07.2020 22:01


Mathematics, 14.07.2020 22:01


Mathematics, 14.07.2020 22:01

Mathematics, 14.07.2020 22:01


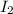 = 1.3 mol
= 1.3 mol = 1.0 mole
= 1.0 mole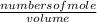
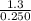
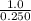
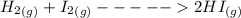
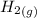 +
+ 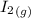 ----->
-----> 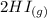
![K = \frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0545/4972/78f4e.png)
![K = \frac{[4-2x]^2}{[x][5.20+x]}](/tpl/images/0545/4972/b30d0.png) where K = 0.983
where K = 0.983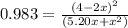
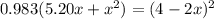
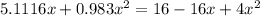
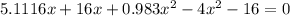
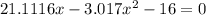
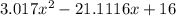
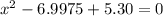
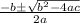
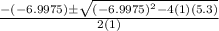
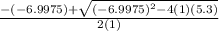 OR
OR