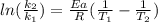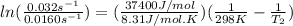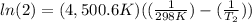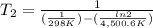Chemistry, 12.03.2020 20:03, shelbylynn17
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s−1 . At what temperature in degrees Celsius would this reaction go twice as fast?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1


Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
Do you know the correct answer?
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s...
Questions in other subjects:










Arts, 02.10.2019 00:30