Chemistry, 12.03.2020 17:25, aubreymoore4553
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressure depression of 0.734 mmHg at 298 ∘C? (The vapor pressure of water at 298 K is 23.76 mmHg.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 00:30, natishtaylor1p8dirz
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
Do you know the correct answer?
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressur...
Questions in other subjects:

Mathematics, 17.12.2020 21:00







Mathematics, 17.12.2020 21:00

Mathematics, 17.12.2020 21:00

Mathematics, 17.12.2020 21:00


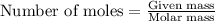
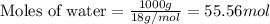
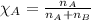
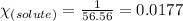
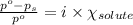
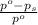 = relative lowering in vapor pressure = 0.734 mmHg
= relative lowering in vapor pressure = 0.734 mmHg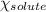 = mole fraction of solute = 0.0177
= mole fraction of solute = 0.0177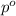 = vapor pressure of pure water = 23.76 torr
= vapor pressure of pure water = 23.76 torr