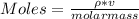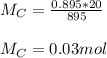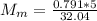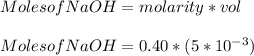You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of moles of vegetable oil, methanol, and NaOH that are initially present in the sample. Assume the density of vegetable oil is 0.895 g/mL and the molar mass is 895 g/mol. Look up the density and molar mass of any other compounds as needed.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 05:10, citlalli30
Name a brittle metal , which is used to galvanize iron
Answers: 1

Chemistry, 23.06.2019 07:30, libbymcvay
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2
Do you know the correct answer?
You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of mol...
Questions in other subjects:

Arts, 19.01.2021 20:00


Mathematics, 19.01.2021 20:00



Mathematics, 19.01.2021 20:00

Mathematics, 19.01.2021 20:00


Social Studies, 19.01.2021 20:00