
Chemistry, 12.03.2020 06:06, amohammad6
A basic solution contains the iodide and phosphate ions that are to be separated via selective precipitation. the i– concentration, which is 9.00×10-5 m, is 10,000 times less than that of the po43– ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of ag3po4, 8.90×10-17.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 06:00, BigGirlsTheBest
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2

Chemistry, 23.06.2019 10:40, 1r32tgy5hk7
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2
Do you know the correct answer?
A basic solution contains the iodide and phosphate ions that are to be separated via selective preci...
Questions in other subjects:



Mathematics, 10.03.2021 21:50



Biology, 10.03.2021 21:50

Health, 10.03.2021 21:50


History, 10.03.2021 21:50


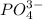 ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of
ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of 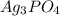 ,
, 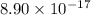 .
.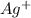 concentration required to cause precipitation of AgI.
concentration required to cause precipitation of AgI.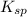 is equal to the ionic product.
is equal to the ionic product.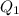 is as follows.
is as follows.![K_{sp} = [Ag^{+}][I^{-}]](/tpl/images/0544/6507/7f793.png)
![[I^{-}] = 7.7 \times 10^{-5}](/tpl/images/0544/6507/26cb8.png)
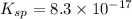
![[Ag^{+}] = \frac{k_{sp}}{I^{-}}](/tpl/images/0544/6507/fac7f.png)
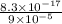
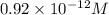
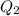 the expression for
the expression for ![K_{sp} = [Ag^{+}]^{3}[PO^{3-}_{4}]](/tpl/images/0544/6507/9aea6.png)
![[Ag^{+}] = (\frac{K_{sp}}{[PO^{3-}_{4}]})^{\frac{1}{3}}](/tpl/images/0544/6507/33b2e.png)
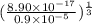
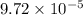 M
M 




