
Chemistry, 12.03.2020 05:41, Rachelmontes1
A 25-kg iron block initially at 350 oC is quenched in an insulated tank that contains 100 kg of water at 18 oC. Assuming the water that vaporizes during the process condenses back in the tank, determine the total entropy change during this process.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1
Do you know the correct answer?
A 25-kg iron block initially at 350 oC is quenched in an insulated tank that contains 100 kg of wate...
Questions in other subjects:



Mathematics, 11.06.2021 20:50


Biology, 11.06.2021 20:50


Mathematics, 11.06.2021 20:50


Mathematics, 11.06.2021 20:50

Mathematics, 11.06.2021 20:50


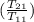 = 25 kg×0.444 J/g·°C㏑
= 25 kg×0.444 J/g·°C㏑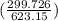 = -8124.296 J/K = -8.124 kJ/K
= -8124.296 J/K = -8.124 kJ/K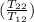 = 100 kg×4.186 J/g·°C㏑
= 100 kg×4.186 J/g·°C㏑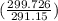 = 12152.01 J/K = 12.15201 kJ/K
= 12152.01 J/K = 12.15201 kJ/K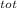 = ΔS₁ + ΔS₂ = -8.124 kJ/K + 12.15201 kJ/K = 4.03 kJ/K.
= ΔS₁ + ΔS₂ = -8.124 kJ/K + 12.15201 kJ/K = 4.03 kJ/K.



