
Chemistry, 12.03.2020 05:32, cxttiemsp021
A compound of molar mass 161 g/mol contains only carbon, hydrogen, bromine, and oxygen. Analysis reveals that the compound contains 12 times as much carbon as hydrogen by mass. Find the molecular formula. Express your answer as a chemical formula.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 19:40, powberier6979
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1


Chemistry, 23.06.2019 10:30, sbelgirl2000
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
Do you know the correct answer?
A compound of molar mass 161 g/mol contains only carbon, hydrogen, bromine, and oxygen. Analysis rev...
Questions in other subjects:

Mathematics, 02.02.2020 05:44

History, 02.02.2020 05:44

Mathematics, 02.02.2020 05:44

Business, 02.02.2020 05:44


History, 02.02.2020 05:44




Mathematics, 02.02.2020 05:44


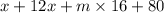 = 161
= 161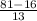
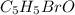 .
.



