
At −11.0 ∘C , a common temperature for household freezers, what is the maximum mass of sucrose (C12H22O11) you can add to 3.00 kg of pure water and still have the solution freeze? Assume that sucrose is a molecular solid and does not ionize when it dissolves in water.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2


Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
Do you know the correct answer?
At −11.0 ∘C , a common temperature for household freezers, what is the maximum mass of sucrose (C12H...
Questions in other subjects:

Mathematics, 02.09.2019 19:30




Computers and Technology, 02.09.2019 19:30

Mathematics, 02.09.2019 19:30





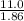 = 5.91 m.
= 5.91 m.
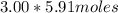 (= 17.73 moles) to the water to get the -11.0° C freezing point depression.
(= 17.73 moles) to the water to get the -11.0° C freezing point depression.





