
Chemistry, 12.03.2020 05:24, redrhino27501
If it takes three "breaths" to blow up a balloon to 1.2 LL, and each breath supplies the balloon with 0.060 moles of exhaled air, how many moles of air are in a 3.0 LL balloon?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Do you know the correct answer?
If it takes three "breaths" to blow up a balloon to 1.2 LL, and each breath supplies the balloon wit...
Questions in other subjects:





Chemistry, 05.11.2020 19:50



English, 05.11.2020 19:50


Mathematics, 05.11.2020 19:50


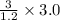 breaths = 7.5 breaths
breaths = 7.5 breaths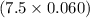 moles of air or 0.45 moles of air.
moles of air or 0.45 moles of air.



