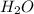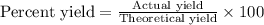Chemistry, 12.03.2020 03:51, brutalgitaffe
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . If of water is produced from the reaction of of sulfuric acid and of sodium hydroxide, calculate the percent yield of water. Round your answer to significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
Do you know the correct answer?
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liqui...
Questions in other subjects:

History, 03.03.2021 18:50

Chemistry, 03.03.2021 18:50

Mathematics, 03.03.2021 18:50

Arts, 03.03.2021 18:50


Mathematics, 03.03.2021 18:50

Mathematics, 03.03.2021 18:50

English, 03.03.2021 18:50

Mathematics, 03.03.2021 18:50


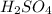 = 72.6 g
= 72.6 g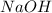 = 77.0 g
= 77.0 g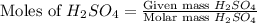




 moles of
moles of