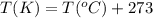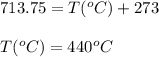Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
Do you know the correct answer?
A sample of gas has a volume of 1.24 L under 2.35 atm pressure at 45°C. If the gas is then expanded...
Questions in other subjects:








Geography, 25.04.2021 01:00

Mathematics, 25.04.2021 01:00


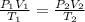
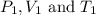 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas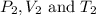 are the final pressure, volume and temperature of the gasW
are the final pressure, volume and temperature of the gasW![P_1=2.35atm\\V_1=1.24L\\T_1=45^oC=[45+273]K=318K\\P_2=0.515atm\\V_2=12.7L\\T_2=?](/tpl/images/0544/2731/0dccc.png)