
Chemistry, 12.03.2020 03:28, kmcgregor4155
Determine the enthalpy for this reaction:
Ca(OH)2(s)+CO2(g)→CaCO3(s)+H2O(l)
Substance ΔHf∘ (kJ/mol)
CO2(g) −393.5
Ca(OH)2(s) −986.1
H2O(l) −285.8
CaCO3(s) −1207.0
H2O(g) −241.8

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 01:10, dontcareanyonemo
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3
Do you know the correct answer?
Determine the enthalpy for this reaction:
Ca(OH)2(s)+CO2(g)→CaCO3(s)+H2O(l)
Substa...
Ca(OH)2(s)+CO2(g)→CaCO3(s)+H2O(l)
Substa...
Questions in other subjects:



Social Studies, 13.11.2020 19:10

Mathematics, 13.11.2020 19:10

Social Studies, 13.11.2020 19:10


Business, 13.11.2020 19:10



Chemistry, 13.11.2020 19:10


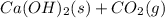 →
→ 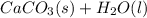
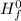 = ( Δ
= ( Δ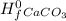 +Δ
+Δ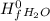 ) - (Δ
) - (Δ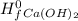 + Δ
+ Δ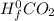 )
)



