
Chemistry, 12.03.2020 01:53, adrian08022
A buffered solution containing dissolved aniline, C 6 H 5 NH 2 , and aniline hydrochloride, C 6 H 5 NH 3 Cl , has a pH of 5.34 . A. Determine the concentration of C 6 H 5 NH 3 in the solution if the concentration of C 6 H 5 NH 2 is 0.245 M. The p K b of aniline is 9.13.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1

Chemistry, 22.06.2019 22:00, luciaaviles3
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 04:31, laurenbreellamerritt
How big are the bighest ocean waves at mavericks
Answers: 1
Do you know the correct answer?
A buffered solution containing dissolved aniline, C 6 H 5 NH 2 , and aniline hydrochloride, C 6 H 5...
Questions in other subjects:

Mathematics, 31.01.2020 19:48




Mathematics, 31.01.2020 19:48

Mathematics, 31.01.2020 19:48


Mathematics, 31.01.2020 19:48



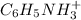 is, 0.0830 M
is, 0.0830 M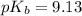
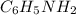 = 0.245 M
= 0.245 M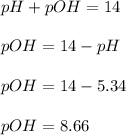
![pOH=pK_b+\log \frac{[Salt]}{[Base]}](/tpl/images/0544/0242/ac570.png)
![pOH=pK_b+\log \frac{[C_6H_5NH_3^+]}{[C_6H_5NH_2]}](/tpl/images/0544/0242/af8e2.png)
![8.66=9.13+\log (\frac{[C_6H_5NH_3^+]}{0.245})](/tpl/images/0544/0242/99488.png)
![[C_6H_5NH_3^+]=0.0830M](/tpl/images/0544/0242/f1802.png)





