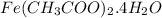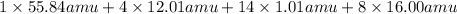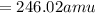Chemistry, 12.03.2020 01:36, Joshuafranklindude
Calculate the molecular (formula) mass of the compound: iron(II) acetate tetrahydrate. A. 186.9 amu B. 245.9 amu C. 191.5 amu D. 242.7 amu E. 197.9 amu

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
Do you know the correct answer?
Calculate the molecular (formula) mass of the compound: iron(II) acetate tetrahydrate. A. 186.9 amu...
Questions in other subjects:

English, 01.02.2021 08:40

Mathematics, 01.02.2021 08:40

English, 01.02.2021 08:40

Mathematics, 01.02.2021 08:40


Mathematics, 01.02.2021 08:40



Mathematics, 01.02.2021 08:40