
Chemistry, 12.03.2020 01:06, bluenblonderw
A solution of I2was standardized with ascorbic acid. Using a 0.1000-g sample of pure ascorbic acid, 25.32 mL of I2 Are required to reach the starch end point. (a)What is the molarity of the iodine solution

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, lizbeth232001
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
Do you know the correct answer?
A solution of I2was standardized with ascorbic acid. Using a 0.1000-g sample of pure ascorbic acid,...
Questions in other subjects:

Mathematics, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20



Mathematics, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20

Biology, 10.12.2020 22:20

World Languages, 10.12.2020 22:20


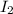 → dehydroascorbic acid +
→ dehydroascorbic acid + 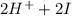
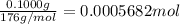
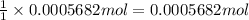 of
of ![[I_2]=\frac{0.0005682 mol}{0.02532 L}=0.02244 M](/tpl/images/0543/9758/3370c.png)




