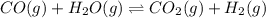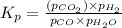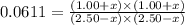Chemistry, 12.03.2020 00:02, Mitchmorgan3816
CO(g ) + H2O(g ) <---> CO2(g ) + H2(g ), Kc = 0.0611 at 2000 K . A reaction mixture initially contains a CO partial pressure of 2.50 atm, an H2O partial pressure of 2.50 atm, a CO2 partial pressure of 1.00 atm, and an H2 partial pressure of 1.00 atm at 2000 K. Calculate the equilibrium partial pressure of CO

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
Do you know the correct answer?
CO(g ) + H2O(g ) <---> CO2(g ) + H2(g ), Kc = 0.0611 at 2000 K . A reaction mixture initially...
Questions in other subjects:











 is the constant of a certain reaction at equilibrium.
is the constant of a certain reaction at equilibrium.